Early reports of patients admitted with COVID-19 identified a high prevalence of liver test abnormalities and an association of these abnormalities with worse clinical outcomes.1,2 To explore the association of liver abnormalities with COVID-19 outcomes, this study examined liver enzyme levels in a population of 37,420 adult patients who were admitted for COVID-19 and had either been discharged or died, as of July 8, 2020. Patients with severely elevated liver enzymes during admission for COVID-19 showed 2.5 times higher mortality rates when compared to patients with normal or moderately elevated levels.
Elevated liver enzymes most commonly indicate acute inflammation of the liver, which may result from direct injury to the liver by infection or from inflammation caused by other physiologic processes, such as bloodstream infection or reduced oxygen levels in the blood. This study assessed levels of two common liver enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and total bilirubin up to 14 days prior to the time of initial admission and at the peak value during hospitalization. Results were classified as normal, moderately elevated, or severely elevated. To reduce survival bias, the study sample excluded patients with multiple COVID-19 admissions. Additionally, the study excluded 1,704 (4%) adult patients who were admitted for COVID-19 but did not have a known outcome as of July 8, 2020.
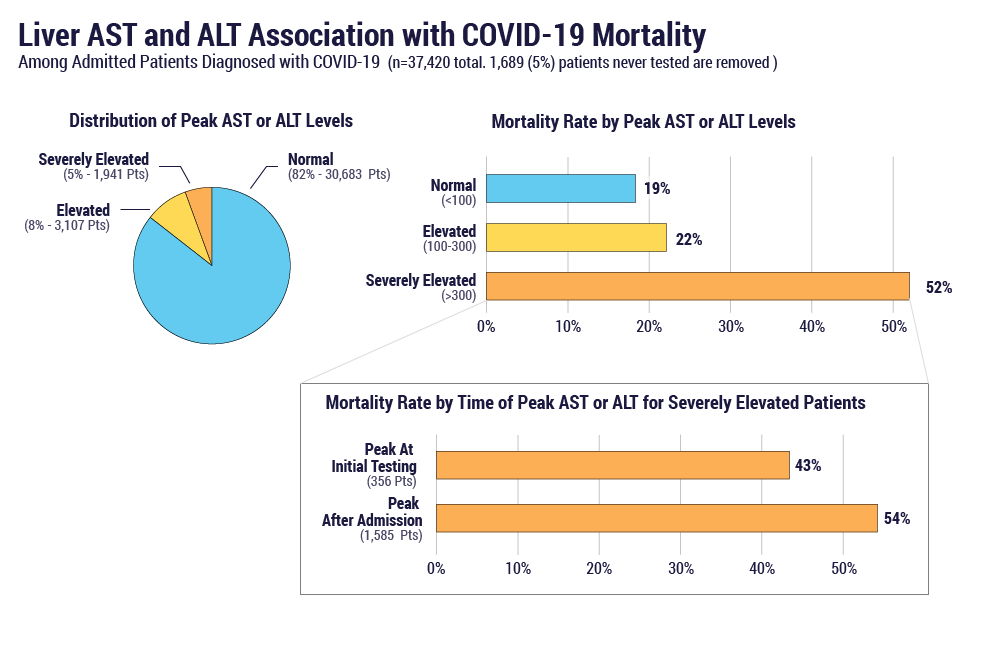
The overall mortality rate in patients admitted for COVID-19 was 20% (7,643/37,420). As shown in Figure 1, patients with severely elevated liver AST or ALT levels at any time during admission were found to have higher mortality rates (52%) than those with normal or moderately elevated results (19% and 22%, respectively). AST levels showed a slightly higher association with increased mortality than ALT or bilirubin. The highest mortality rates (54%) were observed in patients whose levels were normal or moderately elevated at initial presentation and then rose to severe elevations during hospitalization, compared to patients with severe elevations at initial presentation.
In the case of chronic liver disease, liver enzyme elevations do not reliably reflect the overall condition of the liver. When patients with documented pre-existing liver disease were analyzed in this population, they also exhibited higher mortality rates compared to the overall population (26% and 20%, respectively), but this difference was less dramatic than the pattern observed in patients with severely elevated enzyme levels.
Medication toxicity is one potential explanation for the liver enzyme elevations we observed. A study by Cai et al.1 reported liver injury as an adverse reaction of lopinavir, an antiviral that was thought to mitigate the effects of COVID-19. We investigated several COVID-19 treatments with possible liver effects to explore their impact on the prevalence, magnitude, and time to peak of liver enzyme abnormalities in this population. In our analysis, the administration of hydroxychloroquine, remdesivir, prednisone, or dexamethasone did not appear to impact liver enzyme elevation compared to the overall population. Lopinavir was also explored, but the number of patients undergoing this treatment in our population was too small (188) to make a meaningful conclusion.
Overall, patients admitted with COVID-19 who exhibited evidence of liver enzyme abnormalities appeared to have higher mortality rates. This is particularly true in cases where liver enzyme elevations increase in severity during the hospitalization. This could reflect direct involvement of the liver in the disease process, or alternatively, the enzyme elevations may be an indicator for other physiologic processes. For example, similar findings were observed during the 2009 influenza A/H1N1 pandemic, where liver test abnormalities were strongly correlated with hypoxia.3 Further research will be helpful to understand the underlying mechanisms of action and potential treatments to improve outcomes in COVID-19 patients with elevated liver enzymes.
This summary examined liver enzyme levels in a population of 37,420 adult patients who were admitted for COVID-19 and had either been discharged or died, as of July 8, 2020. Data are pooled from 38 health systems representing 258 hospitals that span 19 states and cover 135 million patients.
References:
References
- Cai Q, Huang D, Yu H, et al. COVID-19: Abnormal liver function tests. Journal of Hepatology 2020; https://doi.org/10.1016/j.jhep.2020.04.006
- Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet 2020;5:428-30.
- Papic, N., Pangercic, A., Vargovic, M., et al. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza and Other Respiratory Viruses, 2012;6(3):e2-e5.